LCM Biosensor Technologies, Inc.
Next generation metabolic biomarkers sensing technology In Prevention
and Management of Chronic Disease
and Management of Chronic Disease

Just type your contacts
It's totally free
About
LCM Biosensor Technologies is a Life Sciences Deep Tech company developing the new generation technology for non-invasive self-monitoring of metabolic health applicable in healthcare and wellbeing. The product is a wearable device enabled for noninvasive blood glucose monitoring and early prediction Diabetes and related diseases.
Our Mission is to help people predict early and prevent Diabetes and related life-threatening diseases using innovative deep-tech wearable solutions.
Our Mission is to help people predict early and prevent Diabetes and related life-threatening diseases using innovative deep-tech wearable solutions.
Problem
The invention of noninvasive glucose sensing technology is an unsolved technology problem which hinders the progress in the development of next generation healthcare and wellbeing technologies focused on hard-to-heal health challenges including Diabetes Prediction which is one of biggest unsolved world problems.
Solution
We have invented cutting edge non-invasive glucose sensing technology that allows to monitor blood glucose levels by real time measurement of the osmotic pressure of the intercellular substance of the skin epidermis using an osmotic transducer placed on the skin surface.
The revolutionary disruptive technology provides noninvasive continuous concurrent real-time tracking of various biomarkers at the tissue, cellular, and molecular levels, including blood glucose level, calorie intake and others using an optical-osmotic transducer placed on the skin surface.
Patents were filed in multiple countries USA, China, EU, India: US patent app. No.17/747,380 and US Patent No. US 11,925,463 B2 March, 2024.
The revolutionary disruptive technology provides noninvasive continuous concurrent real-time tracking of various biomarkers at the tissue, cellular, and molecular levels, including blood glucose level, calorie intake and others using an optical-osmotic transducer placed on the skin surface.
Patents were filed in multiple countries USA, China, EU, India: US patent app. No.17/747,380 and US Patent No. US 11,925,463 B2 March, 2024.
Status
Our solution has been validated in a proof-of-concept study of over 70+ healthy and diabetic subjects and over 600 tests, including 272 tests with 62 diabetic subjects. The pre-production prototype (TRL7) has been tested and is ready for mass production.
Diabetes Prediction – World’s Biggest Health Challenge.

World diabetic population
2021: 537 million
2030: 643 million (projected)
→ 541 million adults are at increased risk of developing Type 2 Diabetes
→ 88% of patients are unaware that they are pre-diabetic
→ 88% of patients are unaware that they are pre-diabetic
Type 2 Diabetes is a preventable disease – IF detected early, and a needle free glucose sensing technology must be invented for detecting diabetes early.
2021: 537 million
2030: 643 million (projected)
→ 541 million adults are at increased risk of developing Type 2 Diabetes
→ 88% of patients are unaware that they are pre-diabetic
→ 88% of patients are unaware that they are pre-diabetic
Type 2 Diabetes is a preventable disease – IF detected early, and a needle free glucose sensing technology must be invented for detecting diabetes early.
The Product

Provides continuous noninvasive real-time tracking of various metabolic biomarkers at the tissue, cellular and molecular levels:
• Blood glucose;
• Molecular biomarkers: triglycerides, carbohydrates, hyaluronic acid and others;
• Cellular metabolic rate & Calorie intake;
• Blood pressure / Blood Oxygen.
Healthcare and Wellbeing/Healthy Lifestyle applications:
Diagnostic & monitoring biomarkers - self-monitoring, early diagnostics & prevention of diabetes and related chronic diseases:
• metabolic and closely associated cardiovascular and renal;
• breast cancer;
• skin health & aging;
Nutrition as a prevention – monitoring the rate of cellular metabolism and calorie intake.
The Solution: Optical-Osmotic Transducer
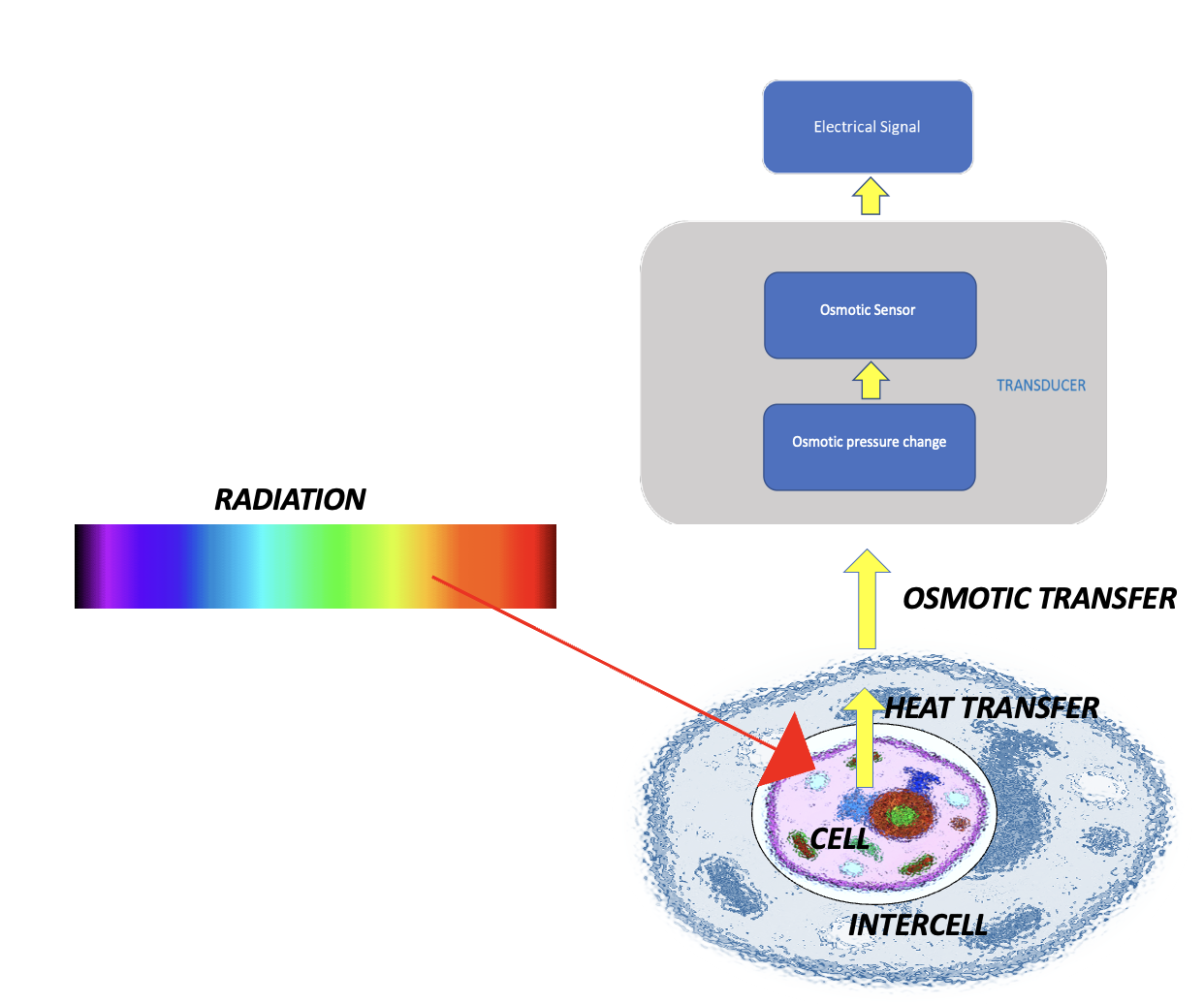
LCM discovered a breakthrough technology that allows to overcome the technological barrier and monitor blood glucose level by noninvasive high-precision measurement
of the osmotic pressure of the intercellular substance of the skin epidermis in real time.
An optical–osmotic transducer placed on the skin surface converts
the change in intercellular osmotic pressure into an electric signal.
of the osmotic pressure of the intercellular substance of the skin epidermis in real time.
An optical–osmotic transducer placed on the skin surface converts
the change in intercellular osmotic pressure into an electric signal.

TECHNOLOGY
A New Approach
We decided to take a different approach by first re-examining the basic physics of glucose interaction with the living tissue. It took years to build a scientific groundwork of Skin Biophysics, that is the Physics of Intercellular Substance – Living Condensed Matter (LCM) Physics which eventually led to the disruptive glucose sensing technology (US patent app. No.17/747,380 and US Patent No.US 11,925,463 B2 March, 2024.
Glucose molecules interact with hyaluronic acid of the extracellular matrix, which leads to the formation of a heterophase structure of an intercellular biopolymer substance that acts as a biosensor that converts changes in glucose concentration into changes in intercellular osmotic pressure.

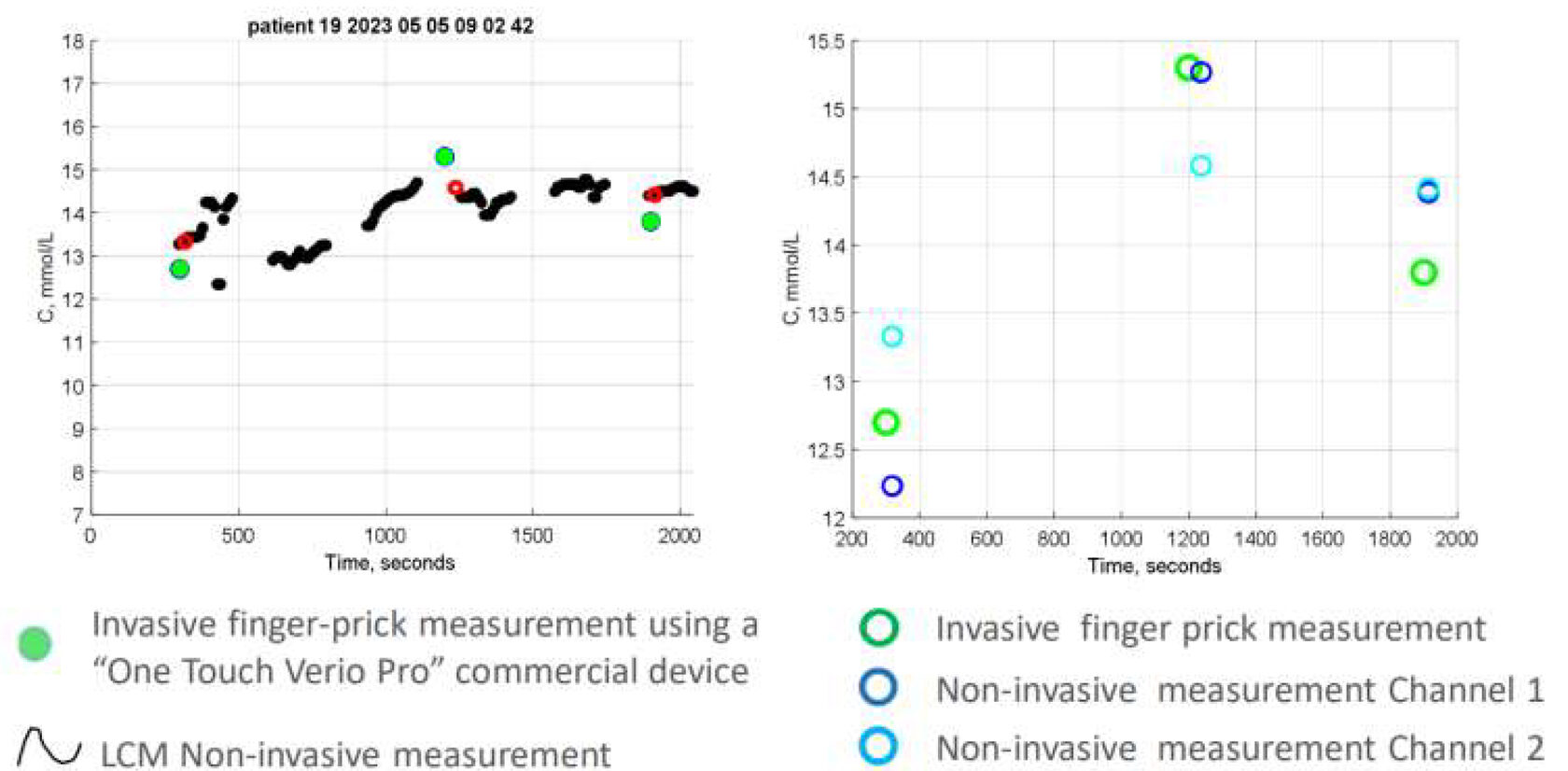
The Prototype – Proof of Concept Clinical Study
The accuracy of LCM measurements is within
FDA-required limits
FDA-required limits
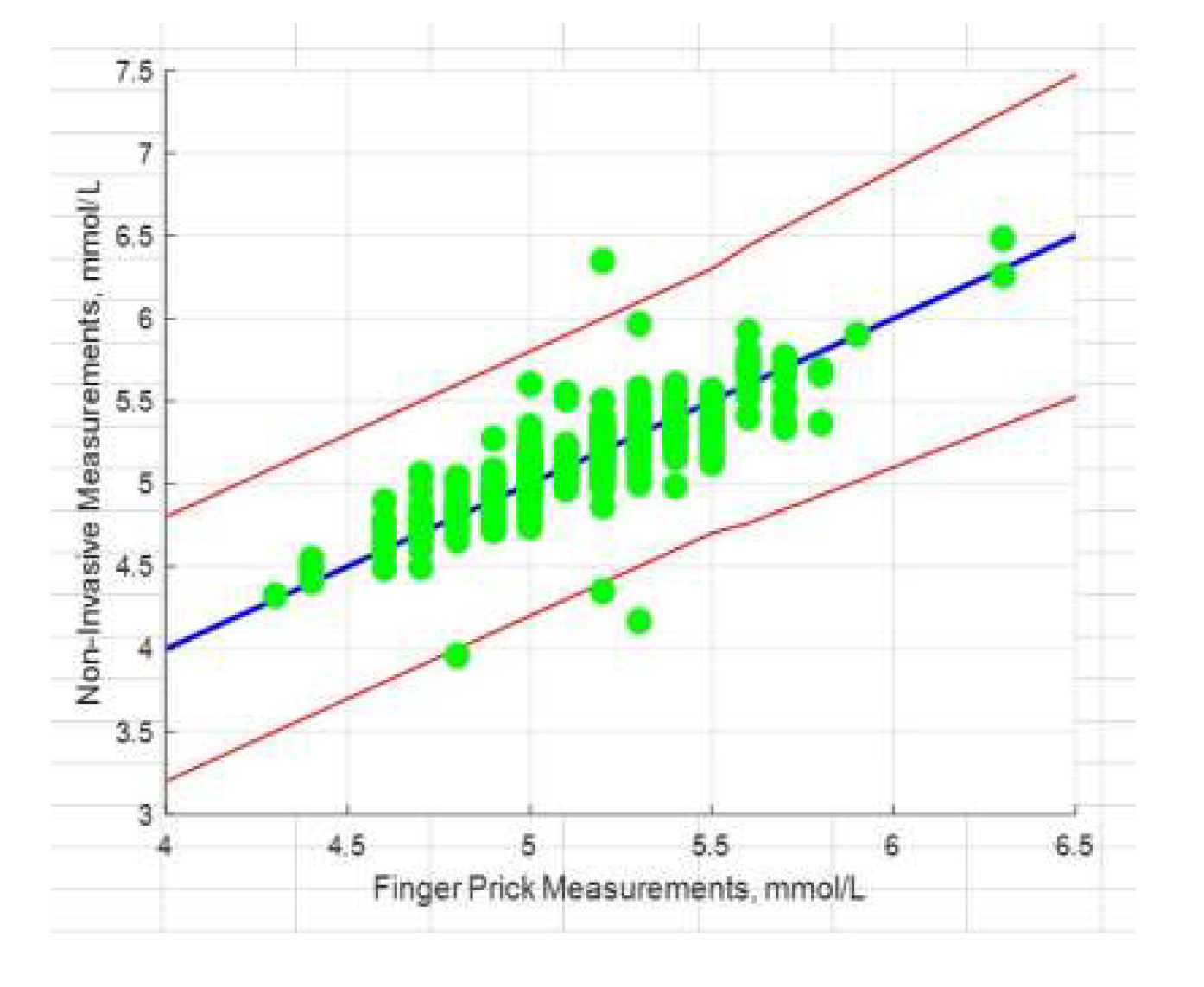
The Prototype – Proof of Concept Clinical Study
Results comparable to an FDA-approved
Results comparable to an FDA-approved
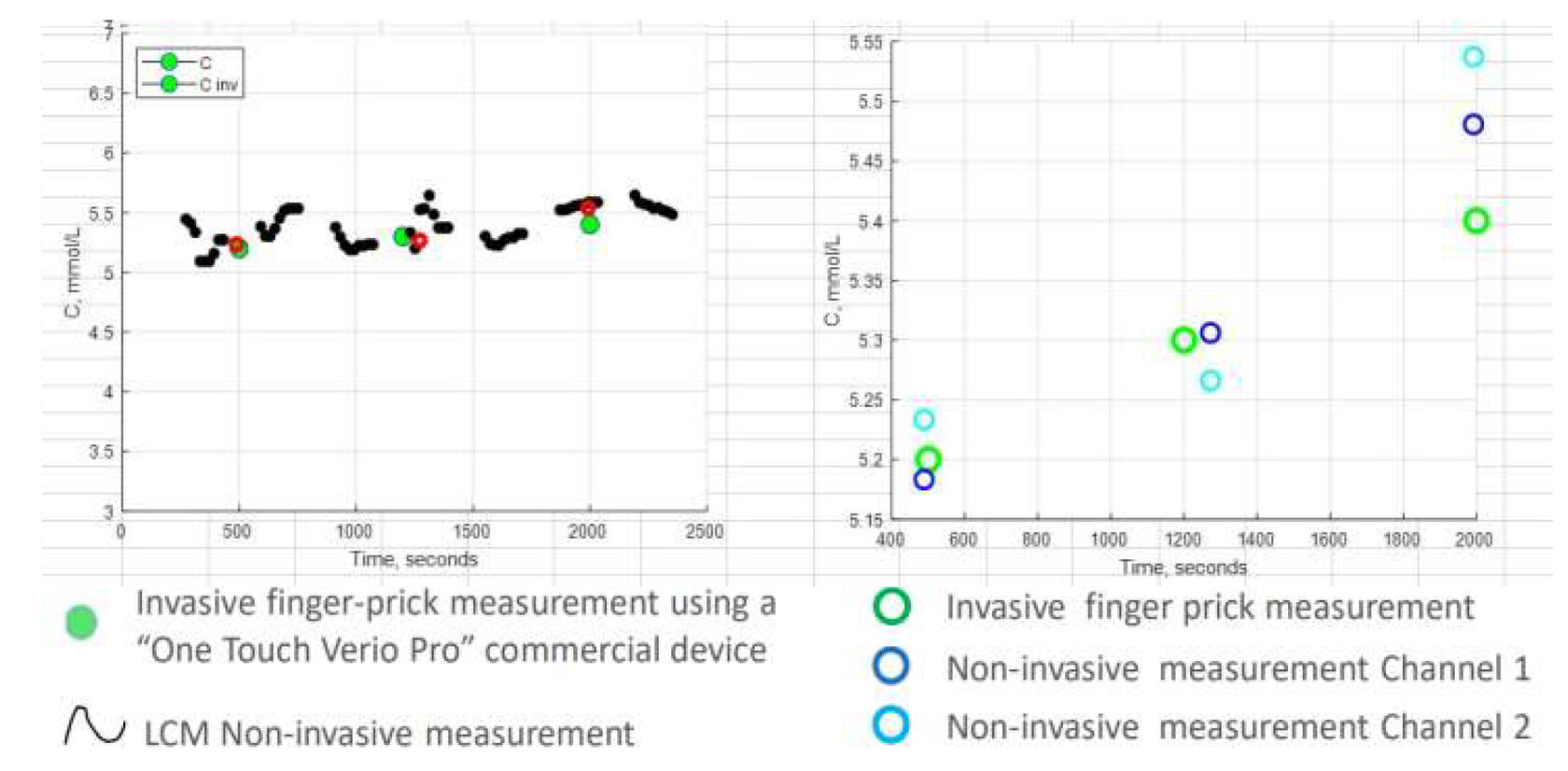
The Prototype – Proof of Concept Clinical Study
Results comparable to an FDA-approved
Results comparable to an FDA-approved
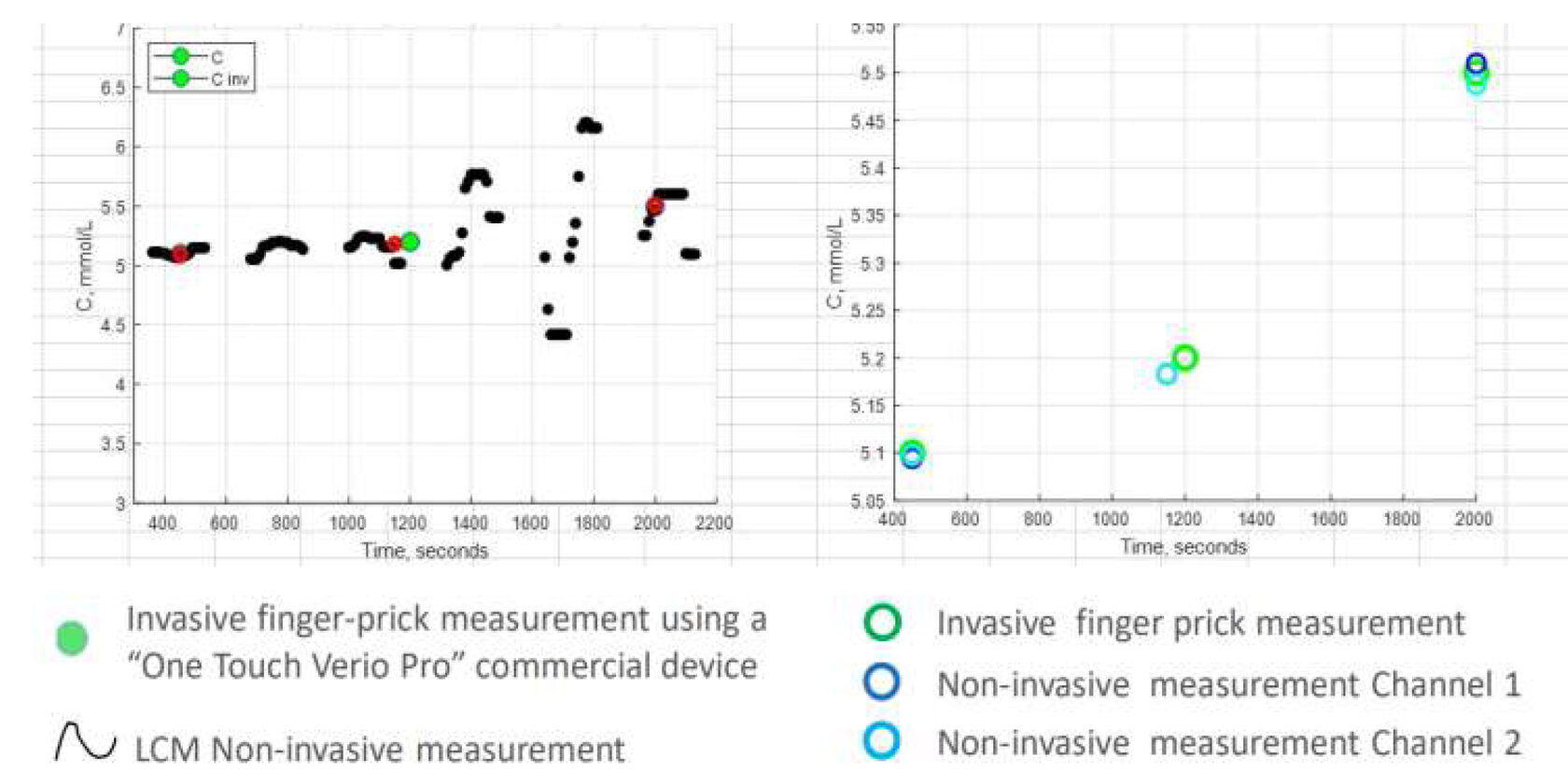
The Prototype – Proof of Concept Clinical Study
Results comparable to an FDA-approved
Results comparable to an FDA-approved
Contact us
Email: info@lcmbiosensor.com
Address: USA 20 Maple Avenue, East Setauket, NY 11733
Address: USA 20 Maple Avenue, East Setauket, NY 11733
